| Get to know Kite's CAR T therapy. |
 |
Developed under the direction and sponsorship of Kite Pharma, Inc. |
|
|
For adult patients with relapsed/refractory (r/r) large B-cell lymphoma (LBCL) after ≥2 lines of systemic therapy: |
 |
BY THE NUMBERS |
|
MORE THAN 3000 PATIENTS HAVE BEEN TREATED WITH YESCARTA®1 |
Across global clinical and commercial settings, thousands of patients with r/r LBCL have received YESCARTA after ≥2 lines of systemic therapy. |
 |
|
|
YESCARTA HAS 3+ YEARS OF CLINICAL EXPERIENCE FROM THE ZUMA-1 PIVOTAL TRIAL2 |
The ZUMA-1 pivotal trial is a phase 2, open-label, single-arm, multicenter trial in 101 adults with r/r aggressive LBCL.2,3 |
|
|
 |
|
|
INDICATION |
YESCARTA is a CD19-directed genetically modified autologous T cell immunotherapy indicated for the treatment of adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy, including diffuse large B-cell lymphoma (DLBCL) not otherwise specified, primary mediastinal large B-cell lymphoma, high grade B-cell lymphoma, and DLBCL arising from follicular lymphoma. |
Limitation of Use: YESCARTA is not indicated for the treatment of patients with primary central nervous system lymphoma. |
IMPORTANT SAFETY INFORMATION |
BOXED WARNING: CYTOKINE RELEASE SYNDROME AND NEUROLOGIC TOXICITIES |
• |
Cytokine Release Syndrome (CRS), including fatal or life-threatening reactions, occurred in patients receiving YESCARTA. Do not administer YESCARTA to patients with active infection or inflammatory disorders. Treat severe or life-threatening CRS with tocilizumab or tocilizumab and corticosteroids. |
|
• |
Neurologic toxicities, including fatal or life-threatening reactions, occurred in patients receiving YESCARTA, including concurrently with CRS or after CRS resolution. Monitor for neurologic toxicities after treatment with YESCARTA. Provide supportive care and/or corticosteroids as needed. |
|
• |
YESCARTA is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the YESCARTA REMS. |
|
Please see additional Important Safety Information below and full Prescribing Information, including Medication Guide. |
|
YESCARTA IS AVAILABLE AT 85+ AUTHORIZED TREATMENT CENTERS (ATCs) NATIONWIDE |
 |
YESCARTA ATCs are located across the country with the number of centers growing on a regular basis. Specialized CAR T healthcare teams at each center are trained on how to infuse YESCARTA and properly monitor and care for patients. |
 |
|
KITE MANUFACTURING IS A RAPID AND RELIABLE PROCESS |
 |
Real-world data has demonstrated a median time of 16 days between leukapheresis and product release.4* |
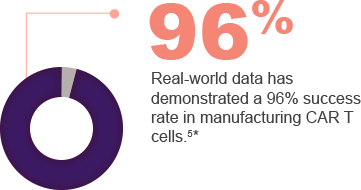 |
*Data as of March 31, 2020. |
 |
|
100% OF TARGETED COMMERCIAL AND MEDICARE PLANS HAVE CONFIRMED COVERAGE FOR YESCARTA6† |
 |
† |
Targeted commercial plans have 100,000 lives or greater. Data as of March 2020. Coverage may vary by patient or by plan. Contact the insurer for more information. |
|
 |
|
|
For adult patients with r/r LBCL after ≥2 lines of systemic therapy |
WHEN YOUR PATIENT RELAPSES, CHOOSE YESCARTA |
|
 |
|
|
IMPORTANT SAFETY INFORMATION (continued) |
CYTOKINE RELEASE SYNDROME (CRS) occurred in 94% of patients, with 13% ≥ Grade 3. Among patients who died after receiving YESCARTA, 4 had ongoing CRS at death. The median time to onset was 2 days (range: 1-12 days) and median duration was 7 days (range: 2-58 days). Key manifestations include fever (78%), hypotension (41%), tachycardia (28%), hypoxia (22%), and chills (20%). Serious events that may be associated with CRS include cardiac arrhythmias (including atrial fibrillation and ventricular tachycardia), cardiac arrest, cardiac failure, renal insufficiency, capillary leak syndrome, hypotension, hypoxia, and hemophagocytic lymphohistiocytosis/ macrophage activation syndrome. Ensure that 2 doses of tocilizumab are available prior to YESCARTA infusion. Following infusion, monitor patients for signs and symptoms of CRS at least daily for 7 days at the certified healthcare facility, and for 4 weeks thereafter. Counsel patients to seek immediate medical attention should signs or symptoms of CRS occur at any time. At the first sign of CRS, institute treatment with supportive care, tocilizumab or tocilizumab and corticosteroids as indicated. |
NEUROLOGIC TOXICITIES occurred in 87% of patients, 98% of which occurred within the first 8 weeks with a median time to onset of 4 days (range: 1-43 days) and a median duration of 17 days. Grade ≥3 occurred in 31% of patients. The most common neurologic toxicities included encephalopathy (57%), headache (44%), tremor (31%), dizziness (21%), aphasia (18%), delirium (17%), insomnia (9%), and anxiety (9%). Prolonged encephalopathy lasting up to 173 days was noted. Serious events including leukoencephalopathy and seizures, as well as fatal and serious cases of cerebral edema have occurred. Following YESCARTA infusion, monitor patients for signs and symptoms of neurologic toxicities at least daily for 7 days at the certified healthcare facility, and for 4 weeks thereafter, and treat promptly. |
REMS: Because of the risk of CRS and neurologic toxicities, YESCARTA is available only through a restricted program called the YESCARTA REMS which requires that: Healthcare facilities that dispense and administer YESCARTA must be enrolled and comply with the REMS requirements and must have on-site, immediate access to a minimum of 2 doses of tocilizumab for each patient for infusion within 2 hours after YESCARTA infusion, if needed for treatment of CRS. Certified healthcare facilities must ensure that healthcare providers who prescribe, dispense, or administer YESCARTA are trained about the management of CRS and neurologic toxicities. Further information is available at www.YESCARTAREMS.com or 1-844-454-KITE (5483). |
HYPERSENSITIVITY REACTIONS: Allergic reactions, including serious hypersensitivity reactions or anaphylaxis, may occur with the infusion of YESCARTA. |
SERIOUS INFECTIONS: Severe or life-threatening infections occurred. Infections (all grades) occurred in 38% of patients. Grade ≥3 infections occurred in 23% of patients; those due to an unspecified pathogen occurred in 16% of patients, bacterial infections in 9%, and viral infections in 4%. YESCARTA should not be administered to patients with clinically significant active systemic infections. Monitor patients for signs and symptoms of infection before and after infusion and treat appropriately. Administer prophylactic anti-microbials according to local guidelines. Febrile neutropenia was observed in 36% of patients and may be concurrent with CRS. In the event of febrile neutropenia, evaluate for infection and manage with broad spectrum antibiotics, fluids, and other supportive care as medically indicated. Hepatitis B virus (HBV) reactivation, in some cases resulting in fulminant hepatitis, hepatic failure, and death, can occur in patients treated with drugs directed against B cells. Perform screening for HBV, HCV, and HIV in accordance with clinical guidelines before collection of cells for manufacturing. |
PROLONGED CYTOPENIAS: Patients may exhibit cytopenias for several weeks following lymphodepleting chemotherapy and YESCARTA infusion. Grade ≥3 cytopenias not resolved by Day 30 following YESCARTA infusion occurred in 28% of patients and included thrombocytopenia (18%), neutropenia (15%), and anemia (3%). Monitor blood counts after infusion. |
HYPOGAMMAGLOBULINEMIA and B-cell aplasia can occur. Hypogammaglobulinemia occurred in 15% of patients. Monitor immunoglobulin levels after treatment and manage using infection precautions, antibiotic prophylaxis, and immunoglobulin replacement. The safety of immunization with live viral vaccines during or following YESCARTA treatment has not been studied. Vaccination with live virus vaccines is not recommended for at least 6 weeks prior to the start of lymphodepleting chemotherapy, during YESCARTA treatment, and until immune recovery following treatment. |
SECONDARY MALIGNANCIES may develop. Monitor life-long for secondary malignancies. In the event that one occurs, contact Kite at 1-844-454-KITE (5483) to obtain instructions on patient samples to collect for testing. |
EFFECTS ON ABILITY TO DRIVE AND USE MACHINES: Due to the potential for neurologic events, including altered mental status or seizures, patients are at risk for altered or decreased consciousness or coordination in the 8 weeks following YESCARTA infusion. Advise patients to refrain from driving and engaging in hazardous occupations or activities, such as operating heavy or potentially dangerous machinery, during this initial period. |
ADVERSE REACTIONS: The most common (incidence ≥20%) include CRS, fever, hypotension, encephalopathy, tachycardia, fatigue, headache, decreased appetite, chills, diarrhea, febrile neutropenia, infections-pathogen unspecified, nausea, hypoxia, tremor, cough, vomiting, dizziness, constipation, and cardiac arrhythmias. |
Please see Important Safety Information above and full Prescribing Information, including BOXED WARNING and Medication Guide. |
References: 1. Data on file [1]. Kite Pharma, Inc. 2020. 2. Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20(1):31-42. 3. YESCARTA® (axicabtagene ciloleucel). Prescribing information. Kite Pharma, Inc; 2020. 4. Data on file [2]. Kite Pharma, Inc. 2020. 5. Data on file [3]. Kite Pharma, Inc. 2020. 6. Data on file [4]. Kite Pharma, Inc. 2020. |
YESCARTA, the YESCARTA Logo, KITE, and the KITE Logo are trademarks of Kite Pharma, Inc. GILEAD is a trademark of Gilead Sciences, Inc. |
All other trademarks referenced herein are the property of their respective owners. |
© 2020 Kite Pharma, Inc. All rights reserved. | YESP0663 06/2020 |
|
|
Help
Password Assistance
Privacy Policy |
Unsubscribe from WebMD Professional Clinical Update |
Unsubscribe from all WebMD Professional emails |
WebMD Professional Services
825 Eighth Avenue
New York, NY 10019 |
This promotional communication is provided by WebMD Professional Services.
|
|
|
|