| ILARIS® (canakinumab)—Now Approved for a New Indication |
 |
|
Dear [Dr] [First Name] [Last Name]: |
|
|
|
|
 |
 |
THE ONLY FDA-APPROVED TREATMENT FOR STILL’S DISEASE |
|
ILARIS® (canakinumab) is FDA approved for the treatment of active Still’s disease including AOSD and SJIA in patients aged ≥2 years, making it the only biologic indicated to treat 7 autoinflammatory diseases across Still’s disease and a range of Periodic Fever Syndromes (PFS). |
|
 |
INDICATIONS |
ILARIS® (canakinumab) is an interleukin-1β blocker indicated for the treatment of the following autoinflammatory Periodic Fever Syndromes: |
• |
Cryopyrin-Associated Periodic Syndromes (CAPS), in adults and children aged 4 years and older, including: |
— |
Familial Cold Autoinflammatory Syndrome (FCAS) |
|
— |
Muckle-Wells Syndrome (MWS) |
|
|
|
• |
Tumor Necrosis Factor Receptor Associated Periodic Syndrome (TRAPS) in adults and pediatric patients |
|
• |
Hyperimmunoglobulin D Syndrome (HIDS)/Mevalonate Kinase Deficiency (MKD) in adults and pediatric patients |
|
• |
Familial Mediterranean Fever (FMF) in adults and pediatric patients |
|
ILARIS® (canakinumab) is indicated for the treatment of active Still’s disease, including Adult-Onset Still’s Disease (AOSD) and Systemic Juvenile Idiopathic Arthritis (SJIA) in patients aged 2 years and older. |
IMPORTANT SAFETY INFORMATION |
CONTRAINDICATION |
ILARIS is contraindicated in patients with confirmed hypersensitivity to the active substance or to any of the excipients. |
Please see additional Important Safety Information below. |
|
ILARIS Is Proven Efficacious in Several Clinical Trials in Still’s Disease1 |
|
 |
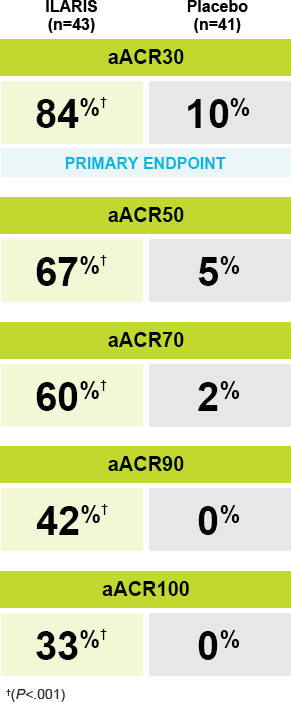
In Study 1: |
Significant improvements in aACR responses were seen with ILARIS at Day 151-3 |
|
 |
|
 |
|
SJIA Study 1 Design1,3 |
|
 |
A randomized, double-blind, placebo-controlled study in 84 patients with SJIA assessed the efficacy of a single subcutaneous dose of ILARIS (4 mg/kg) vs placebo over 29 days. The primary endpoint was aACR30 at Day 15. |
In Study 2 (Part 1): |
|
ILARIS decreased use of steroids within 5 months of treatment1,3 |
|
Of the 92 patients who attempted to taper their corticosteroids, |
 |
62% SUCCESSFULLY TAPERED‡ THEIR STEROID DOSE (n/N=57/92) |
|
|
 |
ALMOST HALF (46%) WERE STEROID FREE (n/N=42/92) |
|
|
SJIA Study 2 Design (Part 1)1,3,4 |
|
 |
An open-label steroid-tapering phase in which 177 patients were treated with a 4-mg/kg subcutaneous dose of ILARIS every 4 weeks for 12 to 32 weeks. Patients receiving concomitant corticosteroids at the beginning of the study were allowed to taper corticosteroid use from Week 9 through Week 28 if they achieved minimum aACR50. |
• |
The primary endpoint was corticosteroid tapering in at least 25% of patients being treated with corticosteroids (45% [57/128] were able to taper their dose of corticosteroids by the end of the steroid-tapering period in Study 2 [Part 1]) |
|
|
|
 |
|
|
 |
Safety profile of ILARIS from core SJIA clinical trials1 |
|
 |
|
|
ILARIS
(n=43) |
Placebo
(n=41) |
|
All infections, %|| |
|
|
Exposure-adjusted incidence rate per 100 patient-days |
|
|
Abdominal pain (upper), % |
|
|
Exposure-adjusted incidence rate per 100 patient-days |
|
|
Mild injection site reaction, % |
|
|
Moderate injection site reaction, % |
|
|
|
|
Corticosteroid-
tapering phase |
ILARIS-
withdrawal phase |
|
ILARIS
(n=177) |
ILARIS
(n=50) |
Placebo
(n=50) |
|
All infections, %|| |
|
|
Exposure-adjusted incidence rate per 100 patient-days |
|
|
Abdominal pain (upper), % |
|
|
Exposure-adjusted incidence rate per 100 patient-days |
|
|
Mild injection site reaction, % |
|
|
Moderate injection site reaction, % |
|
|
|
• |
ILARIS has been associated with an increased risk of serious infections. Infections, predominantly of the upper respiratory tract, in some instances serious, have been reported with ILARIS1 |
|
• |
Generally, the observed infections in ILARIS clinical trials responded to standard therapy. Isolated cases of unusual or opportunistic infections (eg, aspergillosis, atypical mycobacterial infections, cytomegalovirus, herpes zoster) were reported during ILARIS treatment. A causal relationship of ILARIS to these events cannot be excluded1 |
|
• |
Serious infections (eg, pneumonia, varicella, gastroenteritis, measles, sepsis, otitis media, sinusitis, adenovirus, lymph node abscess, pharyngitis) were observed in approximately 4% to 5% (0.02 to 0.17 per 100 patient-days) of patients receiving ILARIS in pivotal studies1 |
|
|
Additional safety for ILARIS throughout SJIA clinical trials1 |
|
No injection site reactions led to study discontinuation |
|
No anaphylactic reactions attributable to treatment with canakinumab were reported |
|
No neutralizing antibodies were detected¶ |
|
|
|
|
 |
ILARIS did not appear to increase the incidence of MAS1: |
• |
Eleven cases of MAS were observed in 201 patients with SJIA treated with ILARIS in clinical trials. Based on the clinical trial experience, ILARIS does not appear to increase the incidence of MAS in Still’s disease patients, but no definitive conclusions can be made |
|
|
• |
MAS is a known, life-threatening disorder that may develop in patients with rheumatic conditions, in particular Still’s disease, and should be aggressively treated |
|
|
* |
aACR response: Percentage improvement (at least 30%, 50%, 70%, 90%, 100%) from baseline in at least 3 of the 6 pediatric ACR core outcome components along with the absence of fever (≤38 °C in the preceding 7 days) and worsening of >30% in no more than 1 of the remaining components. The disease activity components include CRP level, number of joints with active arthritis, number of joints with limited range of motion, physician’s global assessment of disease activity, parent’s or patient’s global assessment of patient’s overall well-being, and functional ability (CHAQ-DI).2,3 |
|
|
‡ |
Successful corticosteroid tapering: Oral prednisone (or equivalent) dose reduction from >0.8 to ≤0.5 mg/kg/day, or from ≥0.5 and ≤0.8 mg/kg/day by at least 0.3 mg/kg/day, or from any initial dose to ≤0.2 mg/kg/day, while maintaining a minimum aACR30 response.2 |
|
|
§ |
The efficacy of ILARIS in adults with AOSD is based on the pharmacokinetic exposure and extrapolation of the established efficacy of ILARIS in SJIA patients. Efficacy of ILARIS was also assessed in a randomized, double-blind, placebo-controlled study that enrolled 36 patients (22 to 70 years old) diagnosed with AOSD. The efficacy data were generally consistent with the results of a pooled efficacy analysis of SJIA patients.1 |
|
|
|| |
The most commonly reported infections were nasopharyngitis and (viral) upper respiratory tract infection. Other infections included pneumonia, rhinitis, pharyngitis, tonsillitis, sinusitis, urinary tract infection, gastroenteritis, and viral infections. |
|
|
¶ |
Antibodies against ILARIS were observed in approximately 3.1% of the patients treated with ILARIS for SJIA. No apparent correlation of antibody development to clinical response or adverse events was observed. |
|
|
aACR=adapted JIA American College of Rheumatology; AOSD=adult-onset Still’s disease; CHAQ-DI=Child Health Assessment Questionnaire-Disability Index; CRP=C-reactive protein; MAS=macrophage activation syndrome; PFS=periodic fever syndromes; SJIA=systemic juvenile idiopathic arthritis. |
|
READY TO HELP TAKE THE FIRE OUT OF AUTOINFLAMMATORY DISEASE? |
|
|
|
|
IMPORTANT SAFETY INFORMATION |
CONTRAINDICATION |
ILARIS is contraindicated in patients with confirmed hypersensitivity to the active substance or to any of the excipients. |
WARNINGS AND PRECAUTIONS |
Serious Infections |
ILARIS has been associated with an increased risk of serious infections. Physicians should exercise caution when administering ILARIS to patients with infections, a history of recurring infections or underlying conditions, which may predispose them to infections. |
ILARIS should not be administered to patients during an active infection requiring medical intervention. Administration of ILARIS should be discontinued if a patient develops a serious infection. |
Infections, predominantly of the upper respiratory tract, in some instances serious, have been reported with ILARIS. Generally, the observed infections responded to standard therapy. Isolated cases of unusual or opportunistic infections (eg, aspergillosis, atypical mycobacterial infections, cytomegalovirus, herpes zoster) were reported during ILARIS treatment. A causal relationship of ILARIS to these events cannot be excluded. In clinical trials, ILARIS has not been administered concomitantly with Tumor Necrosis Factor (TNF) inhibitors. An increased incidence of serious infections has been associated with administration of another interleukin-1 (IL-1) blocker in combination with TNF inhibitors. Coadministration of ILARIS with TNF inhibitors is not recommended because this may increase the risk of serious infections. |
Drugs that affect the immune system by blocking TNF have been associated with an increased risk of new tuberculosis (TB) and reactivation of latent TB. It is possible that use of IL-1 inhibitors, such as ILARIS, increases the risk of reactivation of TB or of opportunistic infections. |
Prior to initiating immunomodulatory therapies, including ILARIS, patients should be evaluated for active and latent TB infection. Appropriate screening tests should be performed in all patients. ILARIS has not been studied in patients with a positive TB screen, and the safety of ILARIS in individuals with latent TB infection is unknown. Patients testing positive in TB screening should be treated by standard medical practice prior to therapy with ILARIS. All patients should be instructed to seek medical advice if signs, symptoms, or high risk exposure suggestive of TB (eg, persistent cough, weight loss, subfebrile temperature) appear during or after ILARIS therapy. |
Immunosuppression |
The impact of treatment with anti-IL-1 therapy on the development of malignancies is not known. However, treatment with immunosuppressants, including ILARIS, may result in an increase in the risk of malignancies. |
Hypersensitivity |
Hypersensitivity reactions have been reported with ILARIS therapy. During clinical trials, no anaphylactic reactions attributable to treatment with canakinumab have been reported. It should be recognized that symptoms of the underlying disease being treated may be similar to symptoms of hypersensitivity. If a severe hypersensitivity reaction occurs, administration of ILARIS should be discontinued and appropriate therapy initiated. |
Immunizations |
Live vaccines should not be given concurrently with ILARIS. Prior to initiation of therapy with ILARIS, patients should receive all recommended vaccinations. In addition, because ILARIS may interfere with normal immune response to new antigens, vaccinations may not be effective in patients receiving ILARIS. |
Canakinumab, like other monoclonal antibodies, is actively transported across the placenta mainly during the third trimester of pregnancy and may cause immunosuppression in the in utero exposed infant. The risks and benefits should be considered prior to administering live vaccines to infants who were exposed to ILARIS in utero for at least 4 to 12 months following the mother’s last dose of ILARIS. |
Macrophage Activation Syndrome |
Macrophage Activation Syndrome (MAS) is a known, life-threatening disorder that may develop in patients with rheumatic conditions, in particular Still’s disease, and should be aggressively treated. Physicians should be attentive to symptoms of infection or worsening of Still’s disease as these are known triggers for MAS. Eleven cases of MAS were observed in 201 SJIA patients treated with canakinumab in clinical trials. Based on the clinical trial experience, ILARIS does not appear to increase the incidence of MAS in Still’s disease patients, but no definitive conclusion can be made. |
ADVERSE REACTIONS |
Serious adverse reactions reported with ILARIS in the CAPS clinical trials included infections and vertigo. The most common adverse reactions greater than 10% associated with ILARIS treatment in CAPS patients were nasopharyngitis, diarrhea, influenza, rhinitis, headache, nausea, bronchitis, gastroenteritis, pharyngitis, weight increased, musculoskeletal pain, and vertigo. |
The most common adverse reactions greater than or equal to 10% reported by patients with TRAPS, HIDS/MKD, and FMF treated with ILARIS were injection site reactions and nasopharyngitis. |
The most common adverse drug reactions greater than 10% associated with ILARIS treatment in SJIA patients were infections (nasopharyngitis and upper respiratory tract infections), abdominal pain, and injection site reactions. |
|
ILARIS is a registered trademark of Novartis AG. |
References: 1. ILARIS [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2020. 2. Data on file. CACZ885G2305 SJIA Study 1 Clinical Study Report. Novartis Pharmaceuticals Corporation; 2011. 3. Ruperto N, Brunner HI, Quartier P, et al; PRINTO; PRCSG. Two randomized trials of canakinumab in systemic juvenile idiopathic arthritis. N Engl Med. 2012;367(25):2396-2406. doi:10.1056/NEJMoa1205099. 4. Data on file. CACZ885G2301 SJIA Study 2 Clinical Study Report. Novartis Pharmaceuticals Corporation; 2012. |
|
|
Novartis Pharmaceuticals Corporation |
|
© 2021 Novartis |
1/21 |
T-ILA-1392453 |
|
|
|
|
|